Be prepared for the next epidemic
If we have learned anything from the emerging epidemics of the past few years, it’s that fast and effective vaccine production is crucial to minimize disruption to the global economy and to people’s day-to-day lives. The most obvious is, of course, the COVID-19 pandemic, but SARS, MERS, avian flu, EBOLA, and Zika have all had their own significant impacts.
One positive from the COVID-19 pandemic is that previously overlooked disruptive vaccine technologies have been given the opportunity to shine. Safety and effectiveness are essential considerations for novel vaccines, but the impact of development speed and ease of manufacturing must not be underestimated.
The ability to react quickly to the emergence of new pathogens offers huge benefits to the companies involved.
How can active virosome technology be applied in vaccine development and production?
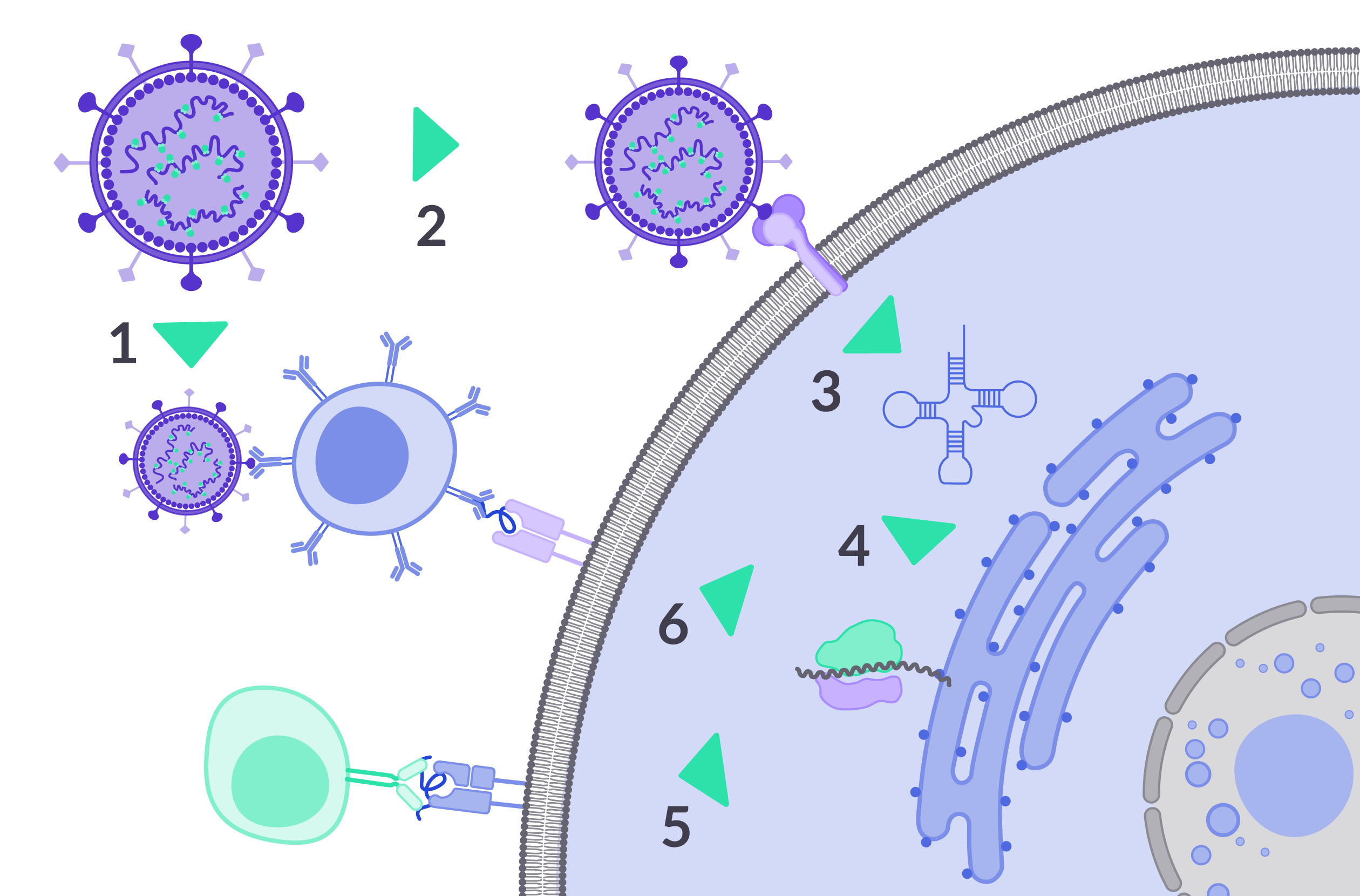
Our vaccine platform has several benefits over the state of the art in vaccine platforms
- Our active virosomes express surface antigens and induce humoral response
- Our active virosomes also carry antigen RNA, and the expressed surface antigen helps the virus to infect the target cells
- Once inside the cells, the RNA self-amplifies…
- …and is translated into proteins by the infected cells
- & 6. Viral antigens are carried to the cell surface by MHC-CL1 (cellular response – 5) and MHC-CL2 (humoral response – 6)
This induces a complete immune response, with both humoral and cellular induction.
How does active virosome technology compare with other vaccine types?
Sagitta’s technology combines the advantages from other technologies without the burdens:
- Rapid development
- Good immunogenicity, with both humoral and cellular immune response
- Non-pathogenic, non-replicative, and no expression of carrier virus antigens
- Possibility of multivalent or single vector vaccines
- Cost-effective development and manufacturing (low OPEX, low CAPEX)
Proof of concept: tetravalent influenza vaccine under development




















Active virosomes can be designed to vaccinate against multiple influenza strains simultaneously by carrying up to 6 foreign genes (17kb) that can be either expressed on the virosome surface and/or injected into target cells.